Fundamental and Bio-Inspired f-Element Science
From the potential contamination of individuals with radioactive material after a nuclear accident to the use of radioisotopes for cancer diagnostics and treatment, the biological chemistry of actinides is relevant to a number of applied problems and reveals outstanding selectivity and specificity features of natural systems for f-element binding. Understanding the fundamental bonding interactions of these selective metal assemblies presents a rich set of scientific challenges as well as opportunities to design new molecular architectures with specific applications in mind. Our research uses a combination of biochemical, spectroscopic, and structural studies in both in vitro and in vivo systems to characterize the selective binding of f-block metal ions by natural and biomimetic ligand architectures.
Systematic and iterative characterization of these selective metal assemblies allows for exploration of the influence of f-orbital bonding on differences in lanthanide and actinide energetic and coordination features, including structural, kinetic, thermodynamic, electrochemical, and optical properties. Harnessing these properties, or combinations thereof, is essential for the development of new therapeutic strategies, environmental decontamination platforms, separations schemes, and diagnostic probes in nuclear medicine. Corresponding structural and spectroscopic data also provide critical benchmarks for the development of theoretical models that may accurately describe and predict f-orbital bonding.
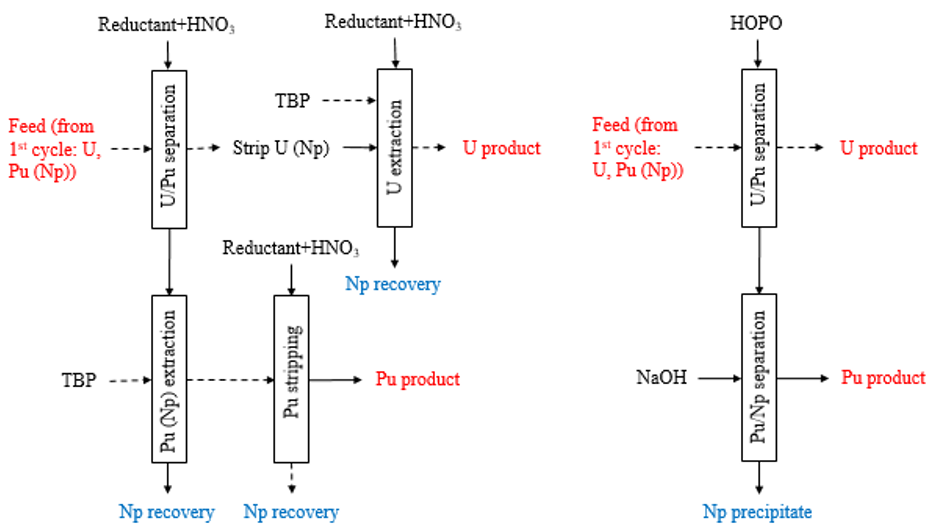
Separations for Spent Nuclear Fuel, Critical Materials, and Medical Isotopes
Various isotopes (cold and radioactive) of the f-elements have become essential for many new energy and health technologies, necessitating new extraction and purification strategies. In addition, expanding the use of nuclear power, through advanced reactor design, new fuel preparation, or spent fuel recycling and/or disposal, presents many separation challenges. Our goal is to delineate fundamental principles that will guide the incorporation of specific molecular systems into higher order materials, ultimately leading to breakthrough and innovation in separation processes. Our work involves various techniques, from tracer-level, radiochemical column separations to large-scale solvent extraction systems, and leverages new computational approaches.
Decorporation
Exposure to radioactive heavy metals in the context of a nuclear accident or attack may have disastrous consequences. We aim to mitigate the toxic effects of internal actinide contamination by using biomimetic synthetic chelators: compounds that exhibit high affinity and specificity for targeted actinides, and complex them so that they may be excreted. Our pre-clinical development program has led to an IND filing for the decorporation agent, 3,4,3-LI(1,2-HOPO), which is formulated as an oral therapeutic. Approval to proceed to first-in-human clinical trials for 3,4,3-LI(1,2-HOPO) was received in August 2014 and initiated the next development phase that will bring this new pharmaceutical to licensure, addressing the need for safety posed by an increasing global use of nuclear energy and the sustained threat of nuclear incidents.
3,4,3-LI(1,2-HOPO) is also being explored as a decorporation agent for residual gadolinium that may be deposited as a result of the use of Gadolinium-Based Contrast Agents (GBCA) during Magnetic Resonance Imaging (MRI) procedures.
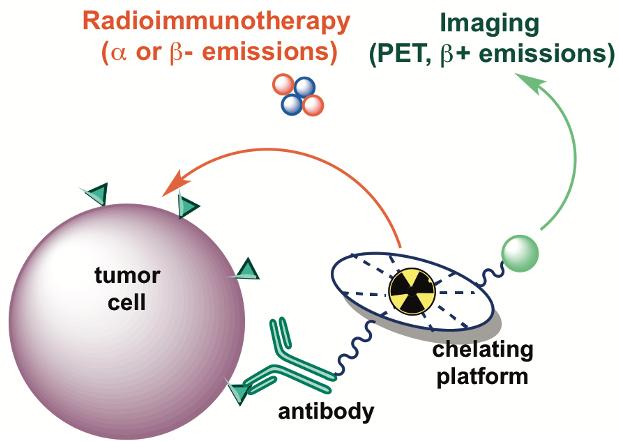
Theranostics
Theragnostics involves using the same pharmaceutical for both therapy and diagnostics. This principle has been applied to radioimmunotherapy, where alpha- and beta-emitting radionuclides are coupled to targeting vectors in order to deliver cytotoxic ionizing radiation to diseased tissue and treat cancer. Before administering these radiopharmaceuticals to a patient, either Positron Emission Tomography (PET) or Single Photon Emission Computed Tomography (SPECT) is used to determine the radiopharmaceuticals’ patient-specific efficacy and dosimetry. As some radiopharmaceuticals do not have suitable nuclear decay properties and therefore cannot be imaged using PET or SPECT, we are evaluating novel imaging radionuclides that can be used as chemical surrogates for therapeutic radionuclides.
Toxicity Pathways and Transport Mechanisms
Actinides and lanthanide fission products, as well as other radionuclides from the nuclear fuel process, are a severe health risk due to radiological and chemical toxicity. The toxicity of these ions is governed by uptake mechanisms, oxidation state and speciation, intracellular distribution, and interactions with various macromolecules, and depends on their physiochemical properties and ligand preferences. Little is known about the toxicity mechanisms of f-block elements at the molecular level, the impact of radiation-induced damage versus chemically-induced damage, and how metal sensing translates into appropriate cellular responses. We are studying actinide and lanthanide speciation and toxicity in both prokaryotes and eukaryotes using a combined functional genomic, biochemical, and spectroscopic approach. Functional genomic analyses include: (i) characterization of gene expression through RNA microarrays and functional gene arrays to capture alterations in genetic regulation, and (ii) characterization of protein regulation and identification of metal-protein complexes using mass spectrometry, fluorescence spectroscopy, radio-detection, liquid chromatography, and gel electrophoresis.
Funding
Our work has been funded by programs from various federal agencies, corporate entities, and private foundations.
Federal
NIH/NIAID
BARDA
NIH/NIBIB
DOE/ECRP
DOE BES HEC
DOE Isotope Production
DOE BES Separations
DOE NEUP
DOE ARPA-E
NSF AI Institutes
DOD CDMRP
Corporate
Bayer AS
AbbVie StemCentrx LLC
Radimmune Therapeutics
Provepharm Inc
Internal
LBNL LDRD Program
LBNL CSR Program
LBNL Innovation Grant Program
Private
Cooley’s Anemia Foundation
Berkeley Lab Foundation
Hellman Fellows Fund
Bakar Fellows Program